AstraZeneca PLC:s resultatrapport för de första nio månaderna och tredje kvartalet 2016
Resultatet för tredje kvartalet är i linje med våra förväntningar

Resultatet för tredje kvartalet är i linje med våra förväntningar

Trial studied Lynparza as maintenance treatment for women with BRCA-mutated metastatic ovarian cancer Initial findings show safety profile with Lynparza tablets was consistent with previous studies

AstraZeneca today announced that the US Food and Drug Administration (FDA) has accepted a complete re-submission of a New Drug Application (NDA) for sodium zirconium cyclosilicate (ZS-9), a potential new medicine for the treatment of hyperkalaemia (high potassium level in the blood serum) by ZS Pharma, a wholly-owned subsidiary of AstraZeneca. The FDA has indicated that this is a complete class 2
AstraZeneca today announced that it has entered into an agreement with Cilag GmbH International, an affiliate of Johnson & Johnson, for the divestment of the rights to Rhinocort Aqua outside the US.
AstraZeneca today announced that it has entered into an agreement with Aralez Pharmaceuticals Trading DAC, a subsidiary of Aralez Pharmaceuticals Inc., for the rights to branded and authorised generic Toprol-XL (metoprolol succinate) in the US
Brilinta did not demonstrate a benefit over clopidogrel in a symptomatic peripheral artery disease patient population
AstraZeneca today announced that MedImmune, its global biologics research and development arm, has entered into a licensing agreement with Allergan plc for the global rights to MEDI2070.
Phase III FALCON trial demonstrates superiority of Faslodex over Arimidex in the 1st-line treatment of hormone-receptor positive (HR+) advanced breast cancer Immuno-Oncology data reinforce commitment to lung and head and neck cancers
AstraZeneca today announced its decision to withdraw the Marketing Authorisation Application (MAA) submitted to the European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) in June 2015 for cediranib in combination with platinum-based chemotherapy
DURATION-8 trial met primary and secondary endpoints, significantly reducing blood sugar (HbA1c), weight and systolic blood pressure versus either medicine alone The first trial to assess GLP-1RA/SGLT-2i combination therapy
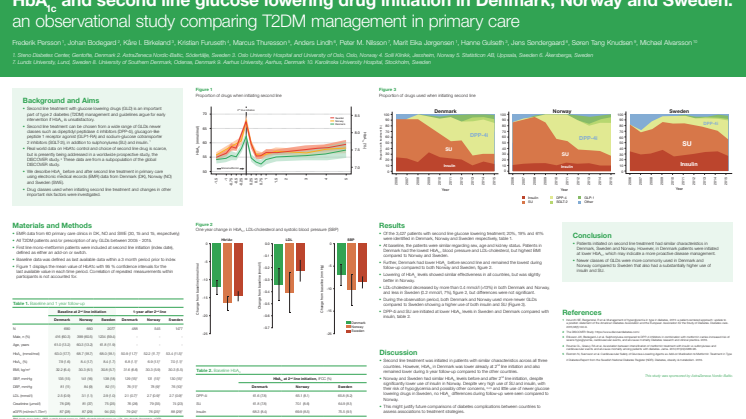
Kontrollen av blodsocker och kolesterol hos patienter med typ 2-diabetes är bättre i Danmark jämfört med i Norge och i Sverige enligt resultat från studien Nordic DISCOVER. Studien är den första nordiska jämförande långsiktiga studien av primärvårdens omhändertagande av patienter med typ 2-diabetes. Resultat presenteras för första gången på den europeiska diabeteskonferensen EASD, 14 september.
Sjukdomskostnaderna för behandling av patienter med typ 2-diabetes fördubblades i Sverige mellan åren 2006 och 2014 samtidigt som kostnaderna för diabetesläkemedel genomgående under perioden endast representerar fyra procent. Det visar resultat från den svenska studien DAISY som presenteras på den europeiska diabeteskonferensen EASD, den 13 september.
First programme to assess potential cardiovascular and renal benefit of an SGLT-2i beyond diabetes
AstraZeneca’s first biologic respiratory medicine met primary and key secondary endpoints in pivotal trials for severe asthma
New evidence to be presented includes pivotal Phase III data on benralizumab
Value-creating divestment supports AstraZeneca’s focus on three main therapy areas Pfizer’s dedicated focus on infectious diseases will extend the reach of the antibiotics to more patients globally and maximise the potential of the late-stage, small molecule antibiotics business
Administration (FDA) Fast Track designation for the development programme in Alzheimer’s disease for AZD3293, an oral beta secretase cleaving enzyme (BACE) inhibitor currently in Phase III clinical trial. The FDA’s Fast Track programme is designed to expedite the development and review of new therapies to treat serious conditions and tackle key unmet medical needs.
AstraZeneca today announced that it has increased its equity interest in Moderna Therapeutics (Moderna) with a $140 million investment as part of Moderna’s preferred-stock financing.
Selumetinib did not meet trial endpoint of progression-free survival in KRASm NSCLC patients.
De totala intäkterna minskade som förväntat med 3%, vilket återspeglar en minskning i produktförsäljning med 2% och är drivet av patentutgångar, i synnerhet Crestor i USA. Merparten av intäkterna från externa samarbeten förväntas ligga under andra halvåret.