We are one of the Biotech Company of the Year finalists at the 2019 European Lifestars Awards!
Scandinavian Biopharma has been nominated to “Biotech company of the year” for the “European Lifestars Awards 2019” by @LSXLeaders.

Scandinavian Biopharma has been nominated to “Biotech company of the year” for the “European Lifestars Awards 2019” by @LSXLeaders.

Vi har framgångsrikt genomfört rekryteringen av den första försöksgruppen (40 vuxna) till Fas I studien i Zambia. Studien är en del av Scandinavian Biopharmas prövningsprogram i Afrika som finansierats med 7,4 miljoner Euro från EDCTP. Utvecklingsprogrammet består av två kliniska studier.

We are proud to announce that the first cohort of 40 adults was enrolled in our Phase I study in Zambia. The study is part of a late phase development program in Africa funded by EDCTP with 7,4 million Euro. The program consists of two clinical trials and is initiated with a Phase I trial in Zambia to evaluate the optimal dose of ETVAX® and the benefits of a booster dose in young children.

Vi har idag uppdaterat Scandinavian Biopharmas varumärkesplattform. Uppdateringen grundar sig i en företagsgemensam djupdykning i Scandinavian Biopharmas affärsmodell, kärnvärderingar och vision.

We have refreshed our brand identity for it to better align with our business model, core values and vision. The whole team has been engaged in the rebranding process and together we have formed the basis of Scandinavian Biopharma’s new graphical design that is being launched today.

Scandinavian Biopharma kommer att delta vid Nordic Life Science Days 2019. Konferensen kommer att äga rum den 10-12 september i Malmö, Sverige. Onsdagen den 11 september, kl 15.30 i rum C, kommer VD Björn Sjöstrand presentera våra framsteg i utvecklingen av världens första diarrévaccin mot ETEC och även berätta om tillväxtresan för vår distributionsverksamhet.

Scandinavian Biopharma will present at the Nordic Life Science Days event 2019. The conference will take place on 10-12 September in Malmö, Sweden. The presentation will be held in Room C at 15:30pm on September 11th and we would like to invite you to listen to an update on progress of the most advanced diarrhoeal vaccine against ETEC and our commercial strategy forward.

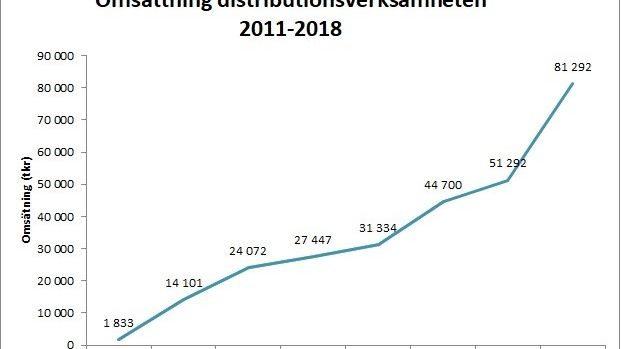
Scandinavian Biopharma can look back on a very successful 2018! The company was granted 7,4 MEUR from EDCTP for testing the protective efficacy of the ETEC vaccine candidate ETVAX® in children below five years of age in Low- and Middle-Income Countries. The distribution business achieved a very strong organic sales growth of 58%.
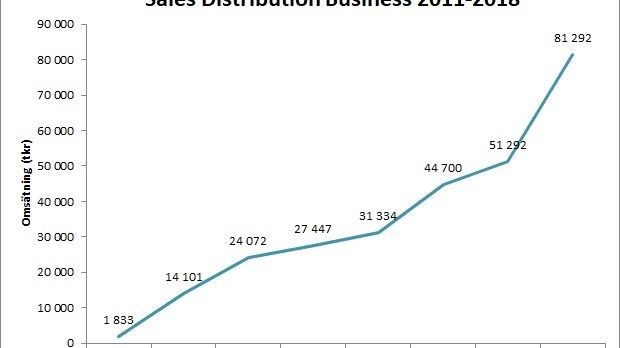
Scandinavian Biopharma kan se tillbaka på ett fantastiskt 2018! Bolaget beviljades 7,4 miljoner Euro från EDCTP för att testa skyddseffekten av ETEC-vaccinet ETVAX® i barn under fem år i låg- och medelinkomstländer. Distributionsverksamheten satte ett nytt omsättningsrekord och växte med 58%. Bolaget och VD Björn Sjöstrand blev uppmärksammade genom utmärkelsen ”Årets företagare i Solna”.
Scandinavian Biopharma continues to expand and has now recruited Dilek Merdol as new head of QC. Dilek has a PhD in infectious biology and has 20 years’ experience in R&D and laboratory operations. She comes from a position as manager in the unit of Clinical Microbiology at Karolinska University Laboratory.
“I am looking forward to the challenges and opportunities that this fast-growing co

Scandinavian Biopharma fortsätter att expandera och har nu rekryterat Dilek Merdol som ny QC chef.

In record time we completed a phase IIB randomized, double-blind, placebo-controlled trial in Finnish travellers going to West Africa. In total 743 travellers spent a fortnight in Benin. Half of the group were given the ETEC vaccine candidate, ETVAX®, and the other half placebo. The aim of the study was to evaluate safety and estimate the protective efficacy in a naive population.

På rekordtid slutförde vi en randomiserad, dubbelblind, placebokontrollerad fas IIB-studie i finländska resenärer till Västafrika. De sista resenärerna lämnade Benin i mars 2019. Totalt har 743 resenärer spenderat två veckor i Benin. Syftet med studien var att utvärdera vaccinets säkerhet och uppskatta den kliniska skyddseffekten i resenärer.

I går, onsdagen den 17 april, invigdes Scandinavian Biopharmas kliniska prövningsprogram i Afrika för bolagets vaccinkandidat ETVAX®. The Permanent Secretary at Ministry of Health, Dr Kenndy Malama invigde tillsammans med Henrik Cederin, Sveriges Ambassadör i Zambia, Dr Izukanji Sikazwe, CEO vid Center for Infectious Disease Research i Zambia (CIDRZ) och Dr Godfrey Biemba, CEO på National Health R

This trial will establish the exact safe and immunogenic dose of the vaccine in young children (6-23 months of age) as well as to explore the potential benefits of a third booster dose. The study will be initiated in healthy adults and followed by young children (6-23 months of age) in Zambia starting from May 2019.

Scandinavian Biopharma continues to expand and now welcomes Madelene Franzén as Accounting Manager. Madelene has previously been an authorized accounting consultant and will be responsible for Scandinavian Biopharma's accounts.

Scandinavian Biopharma fortsätter att expandera och välkomnar nu Madelene Franzén som Accounting Manager. Madelene har tidigare varit auktoriserad redovisningskonsult och kommer att ansvara för Scandinavian Biopharmas redovisning.

In international competition Scandinavian Biopharma has been awarded a grant of 7,4 million Euro for clinical trials of the ETEC vaccine ETVAX®. The trials will be conducted in Zambia and The Gambia. The newly developed vaccine ETVAX® targets diarrhea caused by ETEC – an enteric bacteria responsible for hundred thousands of deaths annually among children in low- to middle income countries.

Scandinavian Biopharma har i tuff internationell konkurrens beviljats ett anslag på 7,4 miljoner Euro för kliniska prövningar av ETEC-vaccinet ETVAX®. Prövningarna kommer genomföras i Zambia och Gambia. ETVAX® är ett nyutvecklat vaccin som avser skydda mot diarré orsakad av ETEC – en bakterie som orsakar hundratusentals dödsfall per år hos barn i låg- och medelinkomstländer.

Scandinavian Biopharma continues to expand and strengthen its organization. Tomas Åslund has now started as VP Manufacturing at Scandinavian Biopharma. Tomas has more than 30 years’ experience from the pharmaceutical and life science industry within the field of recombinant protein drugs.
