News -
Use of TIGR® Matrix Surgical Mesh in Europe off to a roaring start.
Following the announcement of CE mark approval in late August 2011, Novus Scientific has been busily showing off TIGR® Matrix Surgical Mesh to surgeons in many EU countries. The company has also exchanged contracts with several reputable distributors in countries outside of its own geographical reach, and has ensured that regulatory clearance can match supply and demand within the shortest possible time whenever new opportunities arise.
Two newsworthy accounts have already developed in January 2012, namely via Danish distributor KEBOMED, and the HAVEL KLINIK in Germany, the latter of which represents the first use of TIGR® Matrix Surgical Mesh in the German market.
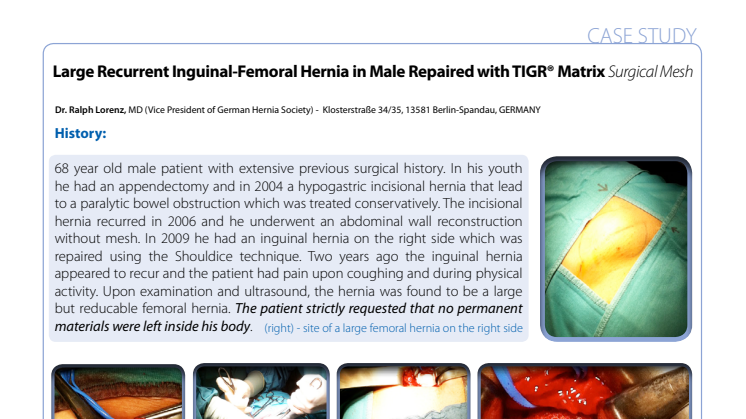
Dr Ralph Lorenz of the Havel Klinik performed a complex femoral hernia recurrence repair on a patient for whom the combination of resorbable materials and most skilled and innovative surgeon were a pre-requisite to undergoing surgery. The surgery was announced a success and the case has been submitted for inclusion in the Novus Scientific case study bank. (available here soon)
Dr Lorenz is a board member of the German hernia society, an organizer of Berliner Hernientage (www.berliner-hernientage.de), runs several educational programs and is extremely highly regarded across Europe and around the world.
For more information regarding the availability and use of TIGR® Matrix Surgical Mesh in your country, please visit the ORDER page of www.novusscientific.com.
Topics
- Health, Health Care, Pharmaceuticals
Categories
- kebo
- havel klinik
- lorenz
- novus scientific
- surgical mesh
- tigr matrix