AstraZeneca reports top-line results from the Brilinta SOCRATES trial in stroke
Missed primary efficacy endpoint; fewer events observed in the Brilinta arm but trend did not reach statistical significance Consistent safety profile
Missed primary efficacy endpoint; fewer events observed in the Brilinta arm but trend did not reach statistical significance Consistent safety profile
AstraZeneca and its global biologics research and development arm, MedImmune, today announced that the US Food and Drug Administration (FDA) has granted Orphan Drug Designation for the investigational anti-CD19 monoclonal antibody,
AstraZeneca today announced that its global biologics research and development arm, MedImmune, has received Fast Track designation from the US Food and Drug Administration (FDA) for its investigational human monoclonal antibody (mAb), MEDI8852, for the treatment of patients hospitalised with Type A strain influenza. The FDA’s Fast Track programme is designed to expedite the development and review
Ltd, för rätten till Moventig (naloxegol) i EU, Island, Norge, Schweiz och Liechtenstein. Moventig är den första orala, perifert verkande my-opioidreceptor-antagonisten (PAMORA) för daglig behandling som är godkänd i Europa för behandling av opioidinducerad förstoppning (OIC) hos vuxna patienter som inte har svarat tillräckligt bra på laxermedel.
Trial didnot meet primaryendpointof improvingoverall survival in challenging to treat mesothelioma patientswith no currently approved treatmentoptions in the second-line setting Tremelimumabremainskeycomponent of Immuno-Oncologycombinationstrategy across multipletumourtypes
Agreement for Plendil in China reinforces AstraZeneca’s focus on innovative medicines
AstraZeneca and Acerta Pharma BV, a company in which AstraZeneca has a majority equity investment, today announced that the European Medicines Agency (EMA) Committee for Orphan Medicinal Products (COMP) adopted three positive opinions recommending acalabrutinib (ACP-196) for designation as an orphan medicinal product.
Godkännande av en ny dos på 60 mg utvidgar den nuvarande indikationen till att även omfatta långsiktig behandling ett år efter tidigare hjärtinfarkt
AstraZeneca today announced that the European Commission (EC) has granted marketing authorisation for ZurampicTM(lesinurad) 200mg in combination with a xanthine oxidase inhibitor (XOI) for the adjunctive treatment of hyperuricemia in adult gout patients (with or without tophi) who have not achieved target serum uric acid (sUA) levels with an adequate dose of an XOI alone.
Zurampic is a selectiv
AstraZeneca and MedImmune, its global biologics research and development arm, today announced that the US Food and Drug Administration (FDA) has granted Breakthrough Therapy Designation (BTD) for durvalumab (MEDI4736),
Two new companies have moved into the BioVentureHub at AstraZeneca Gothenburg, bringing the total to 17 companies and 1 academic group.
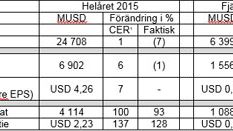
Pascal Soriot, koncernchef, kommenterar resultatet: "Vi stärkte vår forskningsportfölj och levererade ett starkt finansiellt resultat under 2015 samtidigt som vi påbörjade nästa fas av vår strategiska resa. .....
Det första nya läkemedlet som godkänts inom ramen för Europeiska kommissionens accelererade process Godkännandet baseras på studier som visar på en objektiv responsfrekvens av 66 % och en progressionsfri överlevnad med en median på 9,7 månader. Tumörprov eller blodprov kan fastställa vilka patienter som sannolikt kan dra nytta av osimertinib
TAGRISSO™ is the first new medicine to be approved under the European Commission’s expedited process
Approval based on studies showing objective response rate of 66% and median progression-free survival of 9.7 months
Tumour sample or blood test can determine patients likely to benefit from osimertinib
AstraZeneca today announced that the European Commission (EC) has granted conditional m
AstraZeneca today announced that the US Food and Drug Administration (FDA) has granted Breakthrough Therapy designation (BTD) for the oral poly ADP-ribose polymerase (PARP) inhibitor Lynparza™ (olaparib),
AstraZeneca and Incyte Corporation today announced a new collaboration to evaluate the efficacy and safety of Incyte’s Janus-associated kinase (JAK) 1 inhibitor, INCB39110, in combination with AstraZeneca’s next generation epidermal growth factor receptor (EGFR) inhibitor, Tagrisso® (osimertinib). The combination will be assessed as a second line treatment for patients with EGFR mutation-positive
Moderna to lead preclinical development; AstraZeneca to lead clinical development; Moderna to co-commercialise and share profits on resulting products in the US
AstraZeneca, along with its global biologics research and development arm, MedImmune, and Moderna Therapeutics today announced a new collaboration to discover, co-develop and co-commercialise messenger RNA (mRNA) therapeutic candidates
AstraZeneca today announced that the US Food and Drug Administration (FDA) has approved ZURAMPIC®(lesinurad) 200mg tablets in combination with a xanthine oxidase inhibitor (XOI) for the treatment of hyperuricemia associated with gout
AstraZeneca meddelar idag att den europeiska läkemedelsmyndigheten EMA:s rådgivande kommitté CHMP ger positivt besked och rekommenderar godkännande av TAGRISSO™ (AZD9291, osimertinib) och lesinurad, samt ny indikation för BRILIQUE (ticagrelor) 60mg för behandling av patienter som har en historia av hjärtinfarkt och hög risk att få en ytterligare aterotrombotisk händelse.
Durvalumab demonstrated clinical activity and durable responses in 3rd-line or later stage NSCLC patients; full data to be presented at a scientific congress in 2016