Pressmeddelande -
Jonas Bengtsson joins Scandinavian Biopharma as VP Business Development and International Sales
Jonas Bengtsson joins Scandinavian Biopharma with more than 20 years of experience from the vaccine-, pharmaceutical- and medtech-industry including, Sales, Marketing, Business Development and Licensing. His strategic business thinking, his track record of delivering results and his experience around the world are key to accompany Scandinavian Biopharma on its growth path.
Jonas started to work in sales with vaccines for GlaxoSmithKline in 1996. He was in 2001 responsible for establishing the GSK vaccine Business Unit in Sweden including travel, paediatric and flu vaccines. In 2006 Jonas joined SBL vaccines/ Crucell as commercial head of the Nordic, North America and Australia.
In 2010 he joined BSN medical with task to start the Nordic subsidiary. In 2014 he was promoted to SVP Wound Care Vascular in Hamburg. This role included leading global marketing, product development and strategic acquisitions within the franchise.
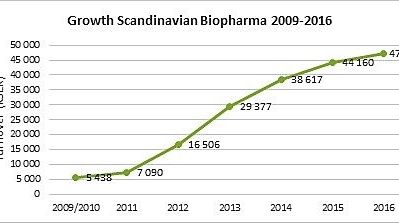
– Scandinavian Biopharma is a very dynamic and fast-growing company. To be part of this professional team will be very exciting and I look forward to add value to the business in the next coming years, says Jonas Bengtsson, who will be responsible for Business Development and International Sales.
– I’m very pleased to welcome Jonas to Scandinavian Biopharma. With his strong track record in the health care industry he will be an important contributor to our continuous growth going forward, says Björn Sjöstrand, CEO Scandinavian Biopharma.
Ämnen
Scandinavian Biopharma
Scandinavian Biopharma is a Swedish research-based biotech company specialist in marketing and sales of vaccines, immunoglobulin’s, biodefense, antidotes and diagnostic tests.
Research and development is mainly focused on development of a new oral traveler’s diarrhea vaccine in collaboration with PATH Vaccine Solutions and University of Gothenburg.