News -
Isansys wearable technology and wireless patient monitoring platform in at-scale deployment at Birmingham Children's Hospital
A ground-breaking project at Birmingham Children’s Hospital is using wireless technology developed by Isansys Lifecare to predict deterioration in seriously ill children and potentially save thousands of young lives.
The project, called RAPID (Real-Time Adaptive & Predictive Indicator of Deterioration), is using biotelemetry and the wireless sensors designed by Isansys Lifecare to collect real-time data on vital signs such as heart rate, breathing rate and oxygen levels. This data is then analysed to predict when a child’s condition may be deteriorating, providing an early warning system that can be acted on immediately.
The project, the first of its type in the world, is jointly funded by a £1.8 million grant from the Wellcome Trust and the Department of Health, through the Health Innovation Challenge Fund, and is on plan to have recruited over 500 patients by April next year and 1,200 patients over the three year lifetime of the project. The RAPID programme is a collaboration between Birmingham Children’s Hospital, Isansys Lifecare, McLaren Applied Technologies, Aston University and the University of Birmingham.
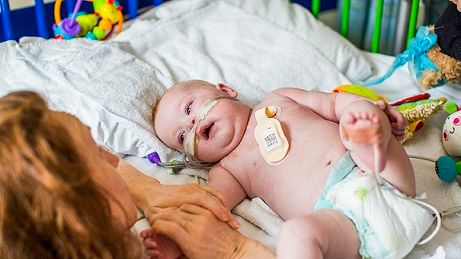
Four-year-old Maci Walford was the first patient to be monitored in the project, which has the potential to revolutionise hospital care. In the case of Maci, who has a congenital heart condition, a set of small high tech wireless sensors, designed by Isansys Lifecare, were attached to her chest and ankle to measure her vital signs continuously. A paediatric version of the Lifetouch™ cardiac sensor was used to collect data directly from the child. The Lifetouch analyses the ECG of every heartbeat to provide continuous heart rate, respiration rate, heart rate variability and, at the push of an on-screen button, a real-time set-up mode ECG trace.
Lifetouch™ is one of the sensors comprising the Patient Status Engine (PSE), a multiple vital sign data capture and analysis system. The data from the Lifetouch™ is transmitted via a low energy Bluetooth connection to the Isansys gateway located near to the bedside. The gateway provides a real-time display of the patient’s vital signs, analyses the data and wirelessly transmits the new clinical information into the Isansys server and from there to the McLaren Applied Technologies system, where the data analytics will run and which delivers the patient information to nurse and clinician dashboards.
Vital signs are normally recorded every one to four hours onto paper charts, but the new RAPID technology enables continuous monitoring and analysis of large amounts of data in real time. This will allow a more accurate prediction of a patient’s deterioration, which is expected to lead to faster and more targeted responses that can save lives and shorten hospital stays. New care pathways enabled by these new technologies need to be evaluated to establish the clinical and quality outcomes as well as the costs and value for money. The University of Birmingham will be doing the Health Economic analysis and providing the biostatistical support for the study.
Dr Heather Duncan, a consultant from Birmingham Children’s Hospital’s Paediatric Intensive Care Unit and leader of the project, said: “This technology is truly transformational. It allows us to analyse many more patients’ data in real-time for the first time in the same way that various other high-risk industries have done for years. The ability to track and identify deterioration towards a cardiac arrest will give doctors the chance to save the patient’s life. I genuinely believe that this will change the way we care for patients in hospital forever.”
Keith Errey, CEO of Isansys Lifecare, said: “We are delighted that our Lifetouch "smart patches” and wireless patient monitoring platform are being deployed in the RAPID project. Following preliminary studies at Birmingham Children’s Hospital and an Innovate UK Smart Award to redesign our technology for paediatric use, it’s wonderful to see it now operational and starting to really help clinicians and nurses improve the lives of young patients and their families.
“This is a very sophisticated patient monitoring system specifically configured for paediatric use which has been delivered as a result of a three-year journey with the Birmingham Children’s Hospital. We are very much looking forward to working closely with the BCH team to optimise the care of critically ill children.
“Monitoring children and neonates is a delicate task, but is one that is absolutely vital. With this technology now available, we’re not just giving families the opportunity to get rid of leads and cables and allow their child to have some freedom whilst being monitored, but we’re also enabling them to know that their child is receiving the best care possible.”
The RAPID project, now moving into its second year, and designed to explore the new clinical pathways and health economic models for the widespread adoption of its methods and technology is based on earlier work:
- July 2011 - First Young Lives study with 3,000 children at Birmingham Children’s Hospital (BCH) demonstrates use of analytics on vital sign data to prevent potential incidents of cardiac arrest.
- June 2013 - Second Young Lives study with 40 children shows use of wireless sensors on wards.
- March 2014 - Isansys completes redesign of Lifetouch for paediatric use, aided by £100,000 Smart Award from Innovate UK.
- Dec 2014 - Isansys awarded £1million contract by theSmall Business Research Initiative (SBRI) to extend Patient Status Engine to include paediatric use and out-of-ward patient monitoring.
Alongside this release, Isansys Lifecare has announced more details on the technology used to support real-time patient monitoring for all patients, including paediatrics. For more details see the release here.
Note to Editors
RAPID is a partnership between Birmingham Children’s Hospital, McLaren Applied Technologies, Aston University, University of Birmingham and Isansys Lifecare Ltd. McLaren Applied Technologies has contributed its unique LIFEINSIGHT platform which processes the data collected in real-time and contains a dashboard to display the relevant information and alerts to hospital staff. Aston University develops and tests the predictive and adaptive algorithm alarm system RAPID, the University of Birmingham ensures the statistics and health outcomes are tracked properly and Isansys Lifecare Ltd are responsible for providing the wireless platform to collect the physiologic data from the patients, and transmit this data to McLaren’s Lifeinsight platform for processing/ analysis and distribution to clinical and nursing staff.
RAPID is funded through the Health Innovation Challenge Fund - a parallel funding partnership between the Wellcome Trust and the Department of Health to stimulate the creation of innovative healthcare products, technologies and interventions and to facilitate their development for the benefit of patients in the NHS and beyond.
http://www.wellcome.ac.uk/Funding/Innovations/Awards/Health-Innovation-Challenge-Fund/index.htm or www.hicfund.org.uk
To find out more about the companies involved in the RAPID project, please click here.