Pressmeddelande -
Scandinavian Biopharma made important progress in research, set a new sales record and strengthened the organization in 2021
2021 was another successful year for Scandinavian Biopharma. The company took important steps in the development of the ETEC vaccine candidate ETVAX® and the distribution business set a new sales record by reaching SEK 93.8 million. The company continued to grow organizationally and expanded the team in both production, QC and QA, this expansion in turn required the business to expand its office space.
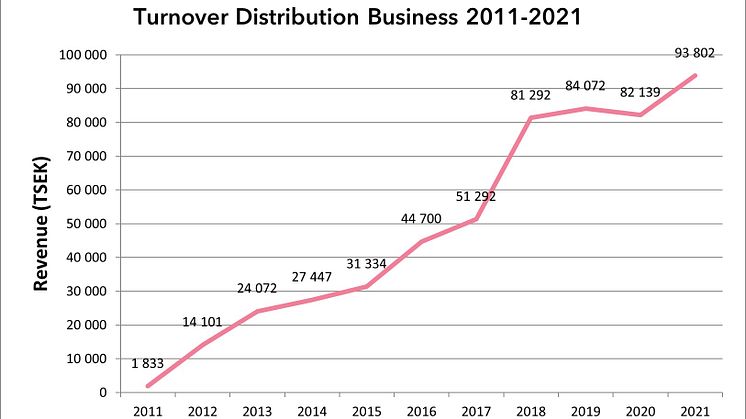
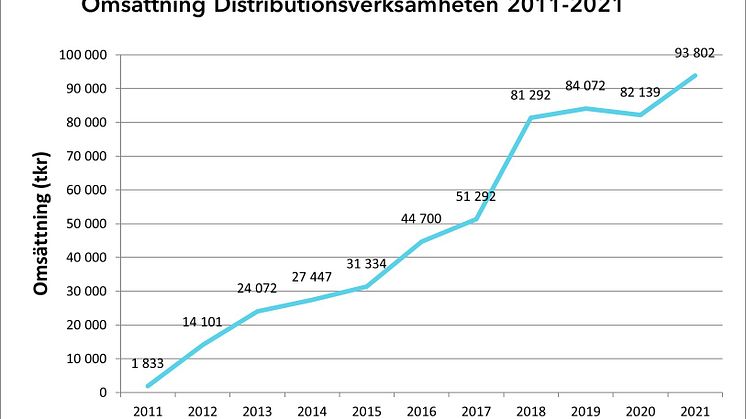
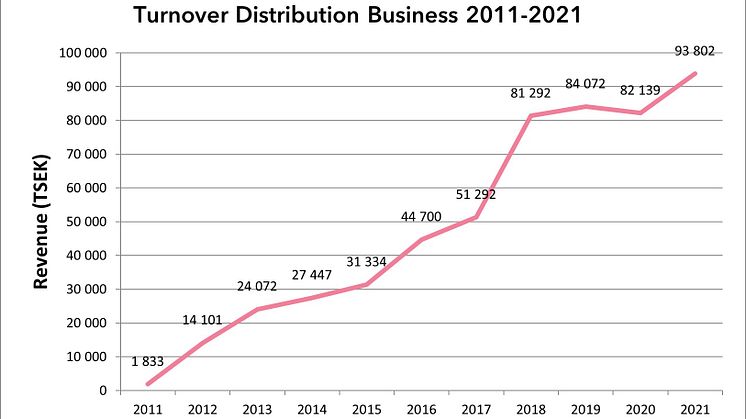
The distribution business set a new record with sales of SEK 93.8 million
The distribution business, which consists of a wide range of specialty biopharma products with a focus on vaccines and immunoglobulins, continued its growth journey in 2021 and sales increased by a full 14%, from SEK 82.1 million in 2020 to SEK 93.8 million in 2021.
"The company's strong growth in 2021 is the result of close cooperation with our partners, both on the supplier and customer side. Sales growth was driven by both new product launches and geographic expansion. We have a strong position in the niche areas in which we operate and look forward with confidence to continued good growth in 2022," says Björn Sjöstrand, CEO of Scandinavian Biopharma.
The clinical development programme for the company's world-leading ETEC vaccine candidate continued to progress well
Positive results from the company's Phase 1 study in Zambia were presented in 2021. Thanks to the study, the company has established the optimal dose of the ETEC vaccine candidate ETVAX® and demonstrated the benefits of a booster dose in children under two years of age. The 246 participants received two vaccine or placebo doses followed by a booster dose.
In 2021, the company also started its large randomised, placebo-controlled and double-blind Phase IIb study in The Gambia, where the overall objective is to investigate the protective effect against moderate to severe diarrhoea caused by ETEC. Just under 4,000 children, aged 6-23 months, have now received at least one dose. All children are scheduled to be fully vaccinated by Q4 2022.
To meet future commercial requirements for launch, the company has further developed the ETEC vaccine candidate formulation. To ensure that the new and more user-friendly formulation produces an equivalent immune response to the previously tested formulation, a comparative immunogenicity study was initiated in 2021 in Gothenburg. This double-blind, randomised Phase II study is planned to be completed in the autumn of 2022.
A major focus in 2021 has also been to build up ETVAX® production capacity with our contract manufacturers in preparation for commercial production.
"In 2021, we have further strengthened our position as a leading diarrhoea vaccine company. We have a very exciting year ahead of us in 2022 as we move towards our goal of developing and registering the world's first vaccine against diarrhoea caused by the ETEC bacteria", says Björn Sjöstrand, CEO of Scandinavian Biopharma.
Ämnen
Kategorier
About Scandinavian Biopharma
Scandinavian Biopharma is a research-based specialty biopharma company determined to give people all over the world a longer and better life. Together with researchers at the University of Gothenburg and the international non-profit organization PATH, Scandinavian Biopharma is developing the first vaccine against ETEC which causes diarrhoea in both travellers and endemic populations.
The development project is mainly funded by the EDCTP programme, which is supported under Horizon 2020, the European Union’s (EU) Framework Programme for Research and Innovation, as well as by PATH and the U.S. Army (USAMMDA).
Scandinavian Biopharma also acts as a distributor for a wide range of specialty biopharma products in Europe with a focus on vaccines and immunoglobulins.
About ETEC
Enterotoxigenic Escherichia coli, or ETEC, is a major cause of bacterial diarrhoeal disease. ETEC infections are particularly common in low- and middle-income countries (LMIC). In children younger than 5 years, it has been estimated that 1.7 billion episodes of diarrhoea occur annually. ETEC represents a major cause of these episodes and accounts for approximately 400 000 deaths among children living in LMICs. In addition, diarrhoea is associated with an increased risk of stunting (i.e. impaired growth and development). Stunting affects cognitive development which might determine a child’s ability to learn, their educational attainment, and future earnings and it also puts children at risk due to other severe infectious diseases.
ETEC is also the leading cause of travellers’ diarrhoea, affecting more than 35 million travellers every year.
About ETVAX®
To date, no vaccine against ETEC exists and ETVAX® is the only ETEC vaccine candidate in late-stage development. The strong immunogenicity and safety data, as well as the promising field efficacy data, clearly distinguish ETVAX® as the lead vaccine candidate against ETEC. Successful development of this vaccine will address a huge unmet medical need for children in LMICs as well as for travellers.