-
-
DATA PRESENTED AT ADA SHOWED THAT BASAL INSULIN THERAPY WITH LANTUS® ACHIEVED TARGET GLYCAEMIC CONTROL IN POORLY CONTROLLED TYPE 2 DIABETES
Two studies show insulin glargine control of HbA1C in poorly controlled type 2 diabetes patients, with comparable rates of hypoglycemia Data presented today at the 65th Session of the American Diabetes Association (ADA), San Diego demonstrated that people with type 2 diabetes achieved significantly better glycaemic control, and also achieve it earlier, when treated with the 24-hour basal insuli
-
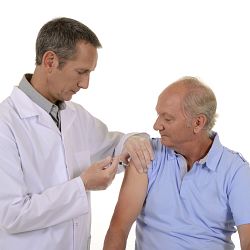
U.S. FDA Licenses sanofi pasteurs ADACEL Vaccine for Combined Protection Against Tetanus, Diphtheria and Pertussis
First and Only Booster in the U.S. for Protection against Pertussis from Adolescence through Adulthood Sanofi pasteur, the vaccines business of the sanofi-aventis Group (NYSE: SNY), announced today that the U.S. Food and Drug Administration (FDA) has licensed ADACEL (Tetanus Toxoid and Reduced Diphtheria Toxoid and Acellular Pertussis Vaccine Adsorbed) Vaccine for protection against tetanus, d
-
SANOFI-AVENTIS JOINS THE PARIS 2012 « CLUB DES ENTREPRISES » BACKING THE PARIS BID TO HOST THE 2012 OLYMPIC AND PARALYMPIC GAMES
Sanofi-aventis, The worlds third largest pharmaceutical group, is joining the Paris 2012 Corporate Club in support of the Paris candidature to host the 2012 Olympic and Paralympic Games. As of today, 20 major companies of international stature - Accenture, Accor, Airbus, Air France, Bouygues, Carrefour, Crédit Agricole, EDF, France Télécom, Gaz de France, Lafarge, Lagardère, LVMH, Publicis, RA
-
ANNUAL GENERAL MEETING
The Annual General Meeting of sanofi-aventis shareholders was held on May 31, 2005. The General Meeting approved the financial statements for the year ended December 31,2004. Also approved was the distribution of a net dividend of 1.20 euro per share, a 17.6% increase over the previous year. The dividend will be paid out on June 7, 2005. The General Meeting has reappointed PricewaterhouseCoope
-
World Heart Federation och sanofi-aventis förenar sina krafter för att förebygga uppkomsten av hjärtkärlsjukdomar
Paris, Frankrike & Genève, Schweiz 23 maj 2005 - World Heart Federation och sanofi-aventis tillkännagav idag att de har ingått ett treårigt partnerskapsavtal för att med förenade krafter förbättra förebyggandet och kontrollen av hjärtkärlsjukdomar. Tillkännagivandet gjordes vid World Heart Federations sjätte internationella kongress om förebyggande kardiologi (ICPC) i Brasilien, och bekräf
-
New study results further enhances the role of Eloxatin® in early and advanced stages of colon cancer
Major data presented at ASCO 2005 on Eloxatin® in combination with other chemotherapeutic drugs and with new targeted agents Paris, France May 18, 2005 - Sanofi-aventis announced today the results of key trials on the anti-cancer drug Eloxatin® (oxaliplatin for injection), presented at the 2005 Annual Meeting of the American Society of Clinical Oncology (ASCO) in Orlando, Florida. The study f
-
Taxotere® regimen demonstrates significant survival benefit in the treatment of advanced gastric cancer
Contact : Anne Bancillon 33-6-86-31-03-89 ~ Taxotere®-Based Chemotherapy Reduces Risk of Death by 23 Percent ~ Final results from the largest international study in the reatment of advanced stomach cancer, also known as gastric cancer, demonstrated that patients who received a Taxotere® (docetaxel) Injection Concentrate-based chemotherapy regimen (Taxotere®, cisplatin and 5-fluorouracil)
-
2005 first-quarter net sales growth ahead of world pharmaceutical
market growth1 at 11.9%2 on a comparable basis Strong growth in adjusted EPS3: up 27.7%2 at 1.06 euros per share Sanofi-aventis on track to meet 2005 guidance The consolidated statement of income of sanofi-aventis for the first quarter of 2005, presented in Appendix 4, shows net income of 531 million euros and is impacted by the accounting treatment of the combination with Aventis and by re
-
1st year results of RIO-EUROPE study published in the Lancet.
Press release from Professor Luc Van Gaal (University Hospital Antwerp), principal investigator of the Rio-Europe trial, entitled: 1st year results of RIO-EUROPE study published in the Lancet. Best regards, Media Relations Department 174, avenue de France - 75013 PARIS - FRANCE - Tél. : +33 1.53.77.40.00 - Fax : +33 1.53.77.41.33 - www.sanofi-aventis.com Sanofi-aventis - Siège Social :
-
Sanofi-aventis affirms its commitment to Access to Medicines in the "Southern countries", with a policy of tiered drug prices geared to populations incomes
Contact : Dr. Robert SEBBAG +33 (0)1 53 77 47 80 +33 (0)6 08 17 21 83 EMBARGO UNTIL APRIL 15, 2005 1:00 p.m. (CET) In his address to the closing plenary session of BioVision the World Life Sciences Forum - Jean-François Dehecq, Chairman and CEO of sanofi-aventis, set out the guiding principles of his Group's strategy in its Policy of Access to Medicines in the "Southern Countries".
-
FDA issues approvable letter for Ambien CR CIV for teh treatment of insomnia
FDA ISSUES APPROVABLE LETTER FOR AMBIEN CR (ZOLPIDEM TARTRATE EXTENDED RELEASE) CIV FOR THE TREATMENT OF INSOMNIA Sanofi-aventis (EURONEXT: SAN and NYSE: SNY) announced today that it has received an approvable letter from the U.S. Food and Drug Administration (FDA) for AMBIEN CR (zolpidem tartrate extended release) CIV. AMBIEN CR is the controlled-release formulation of zolpidem, the worlds
Visa mer
Loading...