Image -
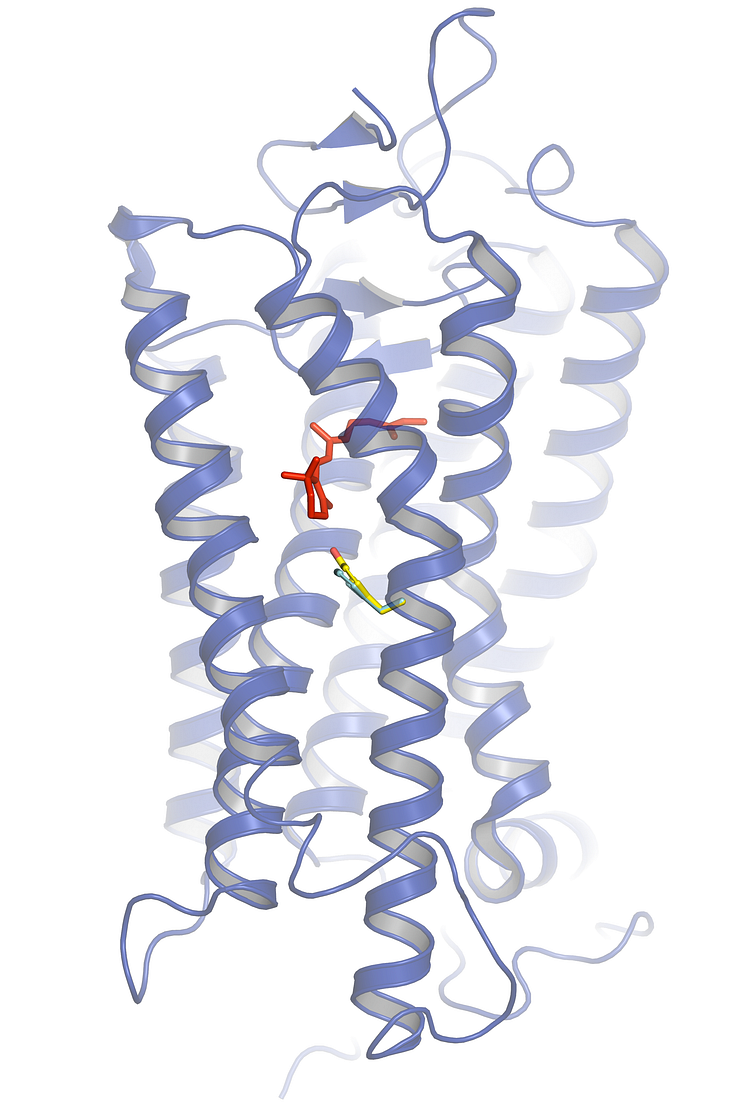
Structural model of herring rhodopsin (blue) with the chromophore retinal in red (where light absorption occurs).
Structural model of herring rhodopsin (blue) with the chromophore retinal in red (where light absorption occurs), the location of residue 261 in the vicinity of retinal, and the tiny but critical difference between the amino acids phenylalanine (turquoise) and tyrosine (yellow/red) highlighted. The presence of tyrosine in Baltic herring rhodopsin makes light absorption red-shifted and better adapted to the Baltic Sea.
Image courtesy Dr. Patrick Scheerer and Dr. Gunnar Kleinau.
- License:
- Media Use
The content may be downloaded by journalists, bloggers, columnists, creators of public opinion, etc. It can be used and shared in different media channels to convey, narrate, and comment on your press releases, posts, or information, provided that the content is unmodified. The author or creator shall be attributed to the extent and in the manner required by good practice (this means, for example, that photographers should be attributed).
- By:
- Image courtesy Dr. Patrick Scheerer and Dr. Gunnar Kleinau.
- File format:
- .png
- Size:
- 2362 x 3543, 3.11 MB