Pressmeddelande -
Tumor Treating Fields prövas vid bukspottkörtelcancer – nu är fas III-studien PANOVA-3 fullrekryterad
Bukspottkörtelcancer, eller pankreascancer, är en svårbehandlad cancerform med mycket dålig prognos. Endast hälften av dem som drabbas är vid liv ett halvår efter diagnos. De stora framsteg som setts inom många andra cancerformer har i stort sett uteblivit när det gäller bukspottkörtelcancer som under 2021 drabbade 1 468 personer i Sverige.
Nu meddelar Novocure att fas III-studien PANOVA-3 är färdigrekryterad. I studien ska Tumor Treating Fieldsmed nab-paklitaxel och gemcitabin prövas vid behandling av lokalt avancerad bukspottkörtelcancer. Det primära effektmåttet i PANOVA-3 är total överlevnad.
Tillägg av TTFields har vid behandling av nydiagnostiserat glioblastom, också en erkänt svårbehandlad tumörform, förbättrat överlevnaden markant. Förhoppningen är nu att liknande förbättringar ska uppnås vid behandling av bukspottkörtelcancer. Slutgiltiga data från PANOVA-3 väntas under 2024.
Novocure has announced that the final patient has been enrolled in the pivotal PANOVA-3 study evaluating the efficacy of Tumor Treating Fields (TTFields) together with nab-paclitaxel and gemcitabine for treatment of patients with locally advanced pancreatic cancer.
“There is a severe unmet need for patients with pancreatic cancer,” said Asaf Danziger, Novocure’s Chief Executive Officer. “Each year in the U.S. alone, approximately 43,000 people are diagnosed with unresectable pancreatic cancer; however, the five-year survival rate remains stagnant at just 10%. The completion of the PANOVA-3 study marks a critical milestone for our company and potentially for patients suffering from this deadly disease. We are proud to be adding to the evolving clinical research and are excited at the prospect of improving patient survival.”
Following the completion of enrollment, an independent Data Monitoring Committee will conduct a pre-specified interim analysis pursuant to the trial protocol. Patients will be followed for a minimum of 18 months.
PANOVA-3 is a randomized, open-label study which was designed to enroll 556 adult patients with unresectable, locally advanced pancreatic adenocarcinoma. Patients have been randomized to receive either the combination of nab-paclitaxel and gemcitabine alone or the combination of nab-paclitaxel and gemcitabine concomitant with TTFields tuned to 150 kHz until progression. The primary endpoint is overall survival. Secondary endpoints are progression free survival, local progression free survival, objective response rate, one-year survival rate, quality of life, pain-free survival, puncture-free survival, resectability rate, and toxicity.
ABOUT PANCREATIC CANCER
Pancreatic cancer is one of the most lethal cancers and is the third most frequent cause of death from cancer in the U.S. While overall cancer incidence and death rates are remaining stable or declining, the incidence and death rates for pancreatic cancer are increasing. It is estimated that approximately 53,000 patients are diagnosed with pancreatic cancer each year in the U.S. Pancreatic cancer has a five-year relative survival rate of just 10%.
Physicians use different combinations of surgery, radiation and pharmacological therapies to treat pancreatic cancer, depending on the stage of the disease. For patients with locally advanced pancreatic cancer involving encasement of arteries but no extra-pancreatic disease, the standard of care is surgery followed by chemotherapy with or without radiation. Unfortunately, the majority of locally advanced cases are diagnosed once the cancer is no longer operable, generally leaving chemotherapy with or without radiation as the only treatment option.
ABOUT TUMOR TREATING FIELDS THERAPY
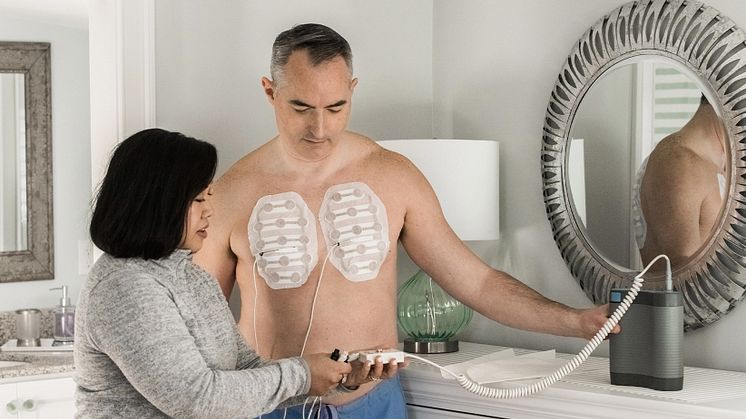
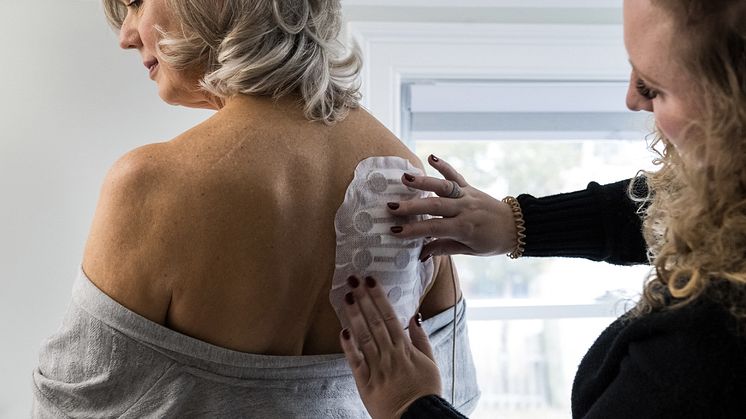
Tumor Treating Fields (TTFields) are electric fields that exert physical forces to kill cancer cells via a variety of mechanisms. TTFields do not significantly affect healthy cells because they have different properties (including division rate, morphology, and electrical properties) than cancer cells. The multiple, distinct mechanisms of TTFields therapy work together to selectively target and kill cancer cells. Due to its multimechanistic actions, TTFields therapy can be added to cancer treatment modalities in approved indications and demonstrates enhanced effects across solid tumor types when used with chemotherapy, radiotherapy, immune checkpoint inhibition, or PARP inhibition in preclinical models. TTFields therapy provides clinical versatility that has the potential to help address treatment challenges across a range of solid tumors. To learn more about Tumor Treating Fields therapy and its multifaceted effect on cancer cells, visit tumortreatingfields.com.
ABOUT NOVOCURE
Novocure is a global oncology company working to extend survival in some of the most aggressive forms of cancer through the development and commercialization of its innovative therapy, Tumor Treating Fields. Novocure’s commercialized products are approved in certain countries for the treatment of adult patients with glioblastoma, malignant pleural mesothelioma and pleural mesothelioma. Novocure has ongoing or completed clinical studies investigating Tumor Treating Fields in brain metastases, gastric cancer, glioblastoma, liver cancer, non-small cell lung cancer, pancreatic cancer and ovarian cancer.
Headquartered in Root, Switzerland and with a growing global footprint, Novocure has regional operating centers in Portsmouth, New Hampshire and Tokyo, as well as a research center in Haifa, Israel. For additional information about the company, please visit Novocure.com and follow @Novocure on LinkedIn and Twitter.
FORWARD-LOOKING STATEMENTS
In addition to historical facts or statements of current condition, this press release may contain forward-looking statements. Forward-looking statements provide Novocure’s current expectations or forecasts of future events. These may include statements regarding anticipated scientific progress on its research programs, clinical study progress, development of potential products, interpretation of clinical results, prospects for regulatory approval, manufacturing development and capabilities, market prospects for its products, coverage, collections from third-party payers and other statements regarding matters that are not historical facts. You may identify some of these forward-looking statements by the use of words in the statements such as “anticipate,” “estimate,” “expect,” “project,” “intend,” “plan,” “believe” or other words and terms of similar meaning. Novocure’s performance and financial results could differ materially from those reflected in these forward-looking statements due to general financial, economic, environmental, regulatory and political conditions as well as issues arising from the COVID-19 pandemic and other more specific risks and uncertainties facing Novocure such as those set forth in its Annual Report on Form 10-K filed on February 23, 2023, and subsequent filings with the U.S. Securities and Exchange Commission. Given these risks and uncertainties, any or all of these forward-looking statements may prove to be incorrect. Therefore, you should not rely on any such factors or forward-looking statements. Furthermore, Novocure does not intend to update publicly any forward-looking statement, except as required by law. Any forward-looking statements herein speak only as of the date hereof. The Private Securities Litigation Reform Act of 1995 permits this discussion.
INVESTORS:
Ingrid Goldberg
investorinfo@novocure.com
610-723-7427
MEDIA:
Leigh Labrie
media@novocure.com
610-723-7428
Ämnen
Kategorier
Novocure är ett onkologiföretag som utvecklar Optune, en behandling som angriper tumörer med Tumor Treating Fields, TTFields. Optume verkar genom elektriska fält som ställs in på specifika frekvenser för att förhindra celldelning i solida tumörer. Optune är idag godkänd för behandling av vuxna patienter med glioblastom. Kliniska prövningar utvärderar Optune vid behandling av hjärnmetastaser, icke-småcellig lungcancer, bukspottkörtelcancer, ovarialcancer och mesoteliom.
Novocure har sin huvudsakliga verksamhet i USA och har kontor i Tyskland, Schweiz, Japan, Israel och Sverige. För ytterligare information om företaget, besök www.novocure.com eller www.twitter.com/novocure.